The PrecivityAD® Blood Test Answers Calls from Patients, Advocates, and Healthcare Providers Who Want Earlier Answers
Overview of the PrecivityAD® Test for Healthcare Providers
Summary of Test and Patient Selection
The PrecivityAD® test identifies whether a patient is likely to have the presence or absence of amyloid plaques in the brain, a pathological hallmark of Alzheimer’s disease. The test relies on precise and robust quantitation of Amyloid Beta 42/40 ratio (Aβ 42/40) and detection of Apolipoprotein E proteotype (equivalent to ApoE genotype) in blood samples, using C₂N’s proprietary mass spectrometry platform.
Additional markers of Alzheimer’s disease, including p-tau217, are currently in development at C₂N’s lab. The PrecivityAD® test is C₂N’s initial test offering; it is expected to be part of a future Brain Health Panel offered by C₂N that will measure multiple pathological markers for Alzheimer’s disease and related disorders.
The PrecivityAD® test is for individuals experiencing memory and thinking issues. The test is only available through a healthcare provider’s order.
The test is available in 49 states, the District of Columbia, and Puerto Rico; the exception is New York, which requires an individual state process for CLIA labs. C₂N is working toward obtaining the requisite certifications that will permit the PrecivityAD® test to be available in New York in the near future. Please periodically refer back to this website or contact us at 1.877.C2N.DIAG (226.3424) for a status update on test availability in New York.
Healthcare Providers: If you are interested in offering the PrecivityAD® test to your patients, please complete the form to start the process for opening a test account and a C₂N representative will be in touch with you shortly.
Requisition forms are included in the PrecivityAD® test kit for signature by healthcare provider and patient.
Our Technology Platform
PrecivityAD® Test Platform: Based on C₂N’s proprietary Stable Isotope Spike Absolute Quantitation (SISAQ™) methodology, the PrecivityAD® test quantifies plasma biomarkers for neurodegenerative conditions using state-of-the-art sample preparation, liquid chromatographic separation, and mass spectrometry quantification technologies.
The SISAQ™ methodology is a proprietary C₂N platform that enables measurement of absolute concentrations of peptides from multiple proteins in biological fluids. This mass spectrometry-based technique identifies and quantifies peptides with a high degree of specificity and precision. Multiple proteomic applications exist for this platform. By multiplexing, it is feasible to measure several biomarkers to create a multianalyte panel of plasma biomolecules (peptides, proteins, lipids, mono- and polysaccharides) that may one day help distinguish among the different classes of neurocognitive disorders. C₂N’s PrecivityAD® blood test is derived from this core platform methodology.
Diagnostic Performance of the PrecivityAD® Test (Aβ42/40 ratio, patient age, and ApoE profile)
In order to clinically validate the PrecivityAD® test’s diagnostic performance, C₂N Diagnostics analyzed blood samples from 686 patients with cognitive impairment and a clinical suspicion of Alzheimer’s disease. All 686 patients had undergone amyloid PET imaging with an FDA-approved amyloid tracer. A total of 378 patients (55%) were amyloid positive by amyloid PET scan. Age was normally distributed with an average age of 73 years, ranging from 60 to 91 years (data on file at C₂N Diagnostics).
The PrecivityAD® test uses liquid-chromatography mass spectrometry to measure the concentration of amyloid beta (Aβ) protein isoforms Aβ42 and Aβ40 and detect the presence of Apolipoprotein E (ApoE)-specific peptides in the blood. The Aβ42 and Aβ40 concentrations are used to calculate the Aβ42/Aβ40 concentration ratio, which is combined with the presence or absence of an ApoE-specific peptide and the age of the patient to provide a numerical result called Amyloid Probability Score (APS). The APS was developed using C₂N ’s proprietary algorithm, and it reflects the likelihood that a patient will be amyloid positive on an amyloid PET scan.
Using data from the 686 patients, C₂N Diagnostics developed an algorithm that identifies amyloid PET status based on plasma Aβ42/40 ratio, ApoE genotype, and patient age. Applying the algorithm to patient data produces an estimated Amyloid Probability Score (APS), which ranges from 0 (low likelihood) to 100 (high likelihood) that the patient will be amyloid positive on amyloid PET imaging.
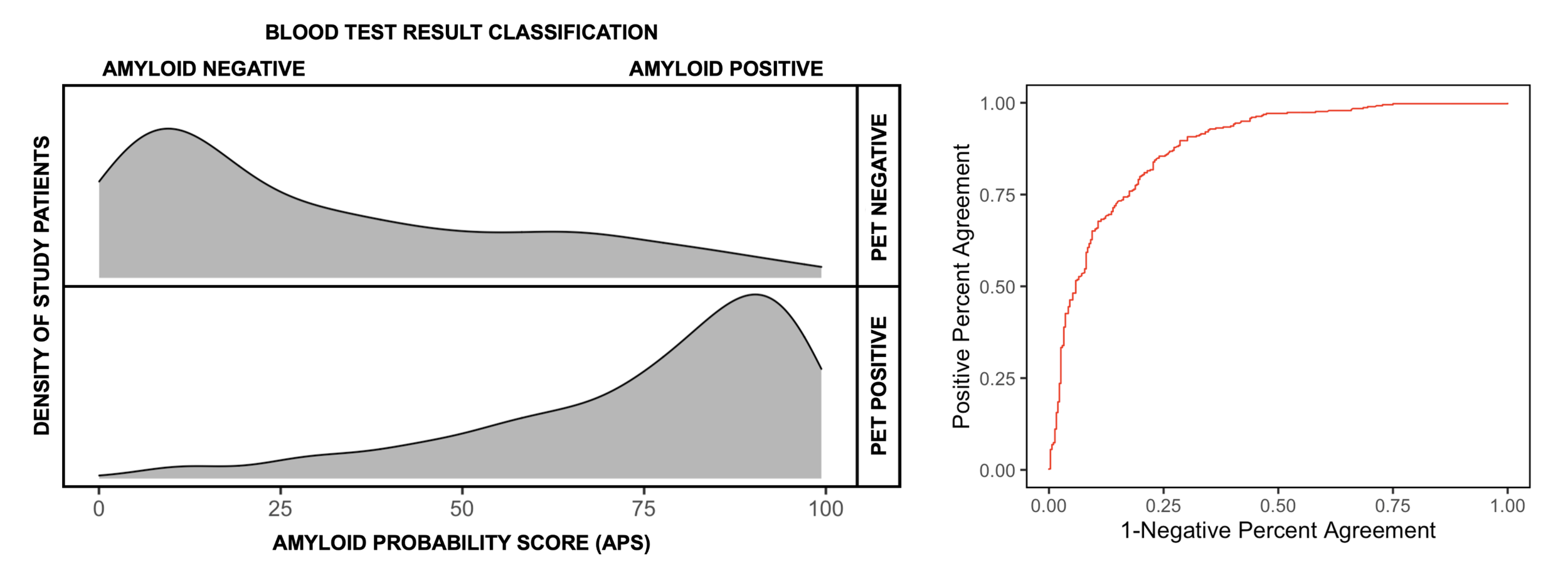
FIGURE 2 (Left): Distribution of Amyloid Probability Scores (APS) grouped by amyloid PET status. (Right) Receiver operating characteristic (ROC) curve with an area under the curve of 0.88.
Figure 2 (left) depicts the APS for each of the patients in the study of 686 patients, stratified by their PET status. In this study, amyloid PET positivity was defined by a quantitative (centiloid) measurement of greater than (>) 25. The Receiver Operating Characteristic (ROC) curve (Figure 2, right) has an area under the curve (AUC) of 0.88. The algorithm’s amyloid assignment confidence increases when two cut points are defined for the test and patients with APS scores above 36 and below 58 are classified as an intermediate category. The selection of these two cut points is based on values that optimize the positive and negative predictive value of PrecivityAD® in a patient population with a 60% prevalence of amyloid PET positivity. With this population prevalence, both the positive and negative predictive value of the test is 86%, and the positive percent agreement (sensitivity) is 92% and negative percent agreement (specificity) is 77%.
Since APS reflects likelihood of the presence or absence of amyloid plaques, higher scores increase the confidence that a patient will be amyloid positive on an amyloid PET scan. Lower scores increase the confidence that a patient will be negative on an amyloid PET scan.
Score Interpretation
The following lists the interpretation of the algorithm’s score:
A Low APS (0 to 35) is consistent with a negative amyloid PET scan result and, thus, a low likelihood of amyloid plaques. Absence of amyloid plaques is inconsistent with an Alzheimer’s disease diagnosis and indicates other causes of cognitive symptoms should be investigated.
An Intermediate APS (36 to 57) does not distinguish between the presence or absence of amyloid plaques and indicates further diagnostic evaluation may be needed to assess the underlying cause(s) for the patient’s cognitive symptoms.
A High APS (58 to 100) is consistent with a positive amyloid PET scan result and, thus, a high likelihood of amyloid plaques. Presence of amyloid plaques is consistent with an Alzheimer’s disease diagnosis in someone who has cognitive decline, but alone is insufficient for a final diagnosis; clinical presentation and other factors should be considered along with APS.
What Our Testing Shows
The PrecivityAD® Results and Interpretation lab report includes:
Amyloid Probability Score (APS): it represents the estimated likelihood from 0 (low likelihood) to 100 (high likelihood) that the patient will be amyloid positive on amyloid PET imaging based on his or her Aβ 42/40 ratio, age and ApoE phenotype. A positive amyloid PET scan is consistent with presence of amyloid plaques and an Alzheimer's disease diagnosis.
In addition to APS, the test report includes the patient’s Aβ 42/40 ratio and ApoE genotype results. Although each of these parameters can individually assess amyloid risk, their use in combination with the patient’s age to calculate the APS is superior, and therefore is the most important result.
The report provides a visual range of Low, Intermediate and High likelihood of amyloid plaques similar to Figure 2. In addition, the report provides an interpretation of the PrecivityAD® test, background and methods.